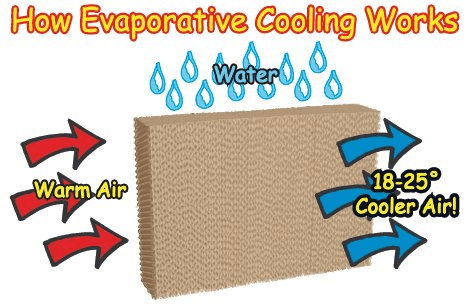
What Is Evaporative Cooling?
When water evaporates from the surface of something, that surface becomes much cooler because it requires heat to change the liquid into a vapor. A nice breeze on a hot day cools us because the current of air makes perspiration evaporate quickly. The heat needed for this evaporation is taken from our own bodies.
As air comes in contact with water it absorbs it. The amount of water absorbed depends largely on how much water is already in the air. The term humidity describes the level of water in the air. If the air holds 50% of its capacity, the humidity would be 50%. If the humidity is low, then the capacity to hold more water is higher, and a greater amount of evaporation takes place.
When the air contains large amounts of moisture, the humidity is said to be high. If the air contains only a small amount of moisture, the humidity is said to be low. When the air holds as much moisture as possible at a certain temperature, the air is saturated. At saturation, the temperature and the dew point are the same. The amount of humidity varies according to the temperature and location. The warmer the air, the more moisture it is able to hold.
The amount of water in the air compared to the amount required for saturation is called relative humidity. If the air contains only half the amount of moisture it can hold when saturated, the relative humidity is 50%.
In order to evaporate water, heat is required. A British Thermal Unit, or B.T.U., is a unit used to measure heat. The evaporation of 1 gallon of water requires almost 8,700 B.T.U.’s of heat. This heat comes from whatever the water is in contact with as it evaporates. This could be a hot sidewalk, a tree, your body, from the air itself, or from wet cooling pads on a Port-A-Cool. As heat is removed from an object, the temperature of that object is decreased, in this case, the air.
The temperature of the water does not have a great effect upon the cooling produced through evaporation. If you placed a gallon of 50° F. water on a warm sidewalk (90° F.), it would produce 9,000 B.T.U.’s of cooling. A gallon of 90° F. water would produce 8,700 B.T.U.’s of cooling, only 3% difference.
The following demonstrates the B.T.U.’s removed from the air based on a given amount of water consumed in an hour:
| 2 gallons……………..17,400 B.T.U.’s removed 3 gallons……………..26,100 B.T.U.’s removed 4 gallons……………..34,800 B.T.U.’s removed 5 gallons……………..43,500 B.T.U.’s removed |
A cubic volume of air at a certain temperature has the ability to hold a certain amount of moisture. In the morning the humidity may be high, but as the day passes and the temperature increases, the relative humidity will naturally decrease. The extent to which relative humidity decreases through the day can be affected by weather systems and proximity to large bodies of water. If a weather system moves in that has a lot of water associated with it already, the drop in humidity will not be as great. Relative humidity drops as air temperature increases. For every 20° rise in temperature, the moisture holding ability of air doubles. For instance, if the temperature of the air was 70° F. and the relative humidity was 100% at 5:00 A.M. and the temperature increased to 90° F. at noon, the moisture holding ability of the air would be twice as much. As a result, the air would now hold only half of the moisture it is capable of holding and the relative humidity of the air would drop to 50%.
The hotter the day, the dryer the air becomes, and the more cooling that can take place through the evaporation of water. When the day gets hot enough to require cooling, the relative humidity will be much lower than in the morning and allow evaporative cooling to work more efficiently.
The efficiency of any evaporative cooling device is directly related to its ability to evaporate water (cool) at a given relative humidity. Units, sometimes described as, “swamp coolers”, with low efficiency will cool only at low relative humidity levels, while a high efficiency unit, such as a Port-A-Cool®, can achieve effective cooling at much higher humidity levels.
History Of The Evaporative Cooling Phenomenon
The ancient Egyptians, Greeks and Romans used wet mats (what we would call cooling pads today) to cool indoor air. They hung the mats over the doors of their tents and other dwellings. When wind blew through the mats, evaporation of the water cooled the air inside. The people of India later used this method to cool the royal palaces.
During the 1500’s the first mechanical fan was built to provide ventilation. In 1800, textile manufacturers in New England began using water evaporative systems to condition the air in their mills. The systems consisted of large “cooling towers” with fans that transported the water-cooled air inside buildings.
In 1939 “swamp cooler” type devices were produced and presented relief from heat for homes and facilities. They cooled the temperature on certain days, when the relative humidity was low, around 8 degrees.
Evaporative Cooling And Port-A-Cool®
Port-A-Cool® portable evaporative cooling units are amazing products, fully developed in 1992, that offer many exciting versatile options for cooling when standard air-conditioning may be unavailable, impractical or cost prohibitive. The units operate at a fraction of the cost of conventional air-conditioning and are capable of cooling thousands of square feet 20 degrees or more, saving Port-A-Cool® customers hundreds, or even thousands of dollars per season!
Since Port-A-Cool® products are state of the art, high efficiency, portable evaporative cooling systems, their high efficiency cooling media coupled with unique housing designs, allow Port-A-Cool® units to cool effectively even in very high humidity conditions, conditions that no other portable evaporative cooling device, such as a “swamp cooler” could possibly approach. Since Port-A-Cool® units are portable, they can be easily moved around shops, factories, back yards, sporting events and hundreds of other areas for maximum effectiveness, comfort and increased productivity.
Port-A-Cool® uses a specially designed ridged cooling media, contained in a properly designed housing to insure effective direction of the air over the water saturated media at the proper air velocity. The effectiveness of Port-A-Cool® products is best appreciated when the temperature is above 95° F. and below 75% relative humidity.